Products
Rare Earth Elements
ABx Rare Earths Strategy
Rare earths have many applications in a wide variety of industries. Permanent magnets are the most valuable application, representing over 90% of the total value of rare earths consumption. Permanent magnets are used in electric vehicles, wind turbines, smartphones and military applications. The four most important rare earths for permanent magnets are neodymium, praseodymium, dysprosium and terbium. Furthermore, the demand for these four ‘supermagnet’ rare earths is predicted to grow faster than for other rare earths. Prices for these rare earths have risen significantly in the last two years (Figure 1).
Globally, most rare earths are sourced from hard-rock mines. These typically require large, costly processing plants and a significant lead time to reach production.
A less common source of rare earths is ionic adsorption clay (IAC) deposits, which have historically been mined only in southern China. A major advantage of IAC deposits is that the rare earths can be extracted from the clay via a low-cost desorption process. Secondly, they often exist at shallow depth. These advantages enable a project to be developed rapidly and at lower cost. Furthermore, IAC deposits typically contain a higher proportion of heavy rare earths compared to hard rock deposits, and low concentrations of radioactive elements such as uranium and thorium.
ABx has discovered rare earth accumulations within our bauxite tenements in northern Tasmania (Figure 2), and is the first company to discover rare earths in Tasmania.
ABx engaged Australian Nuclear Science and Technology Organisation (ANSTO) to conduct desorption tests, which found the highest extractions under relatively neutral conditions reported from any clay-hosted project in Australia[1],[2]. This proves the mineralisation at Deep Leads / Rubble Mound to be of the IAC variety. Low-cost processing is crucial for clay-hosted rare earth deposits, and industry processing experts indicate that low-cost processing can only be achieved using desorption with low acid consumption. This puts ABx at the forefront for investors and countries seeking to diversify rare earths supply.
Following these excellent discovery and processing results, ABx has built significant momentum and will continue to conduct further exploration, which will include targeting new areas within its tenements that have geological features considered prospective for additional rare earths.
The ABx strategy is to produce a mixed rare earth carbonate that can be sold to existing refineries to increase their production. The ABx carbonate will be high in heavy rare earths and low in radioactive elements, which is expected to be attractive to many prospective customers. Market discussions with several potential customers endorse this strategy.
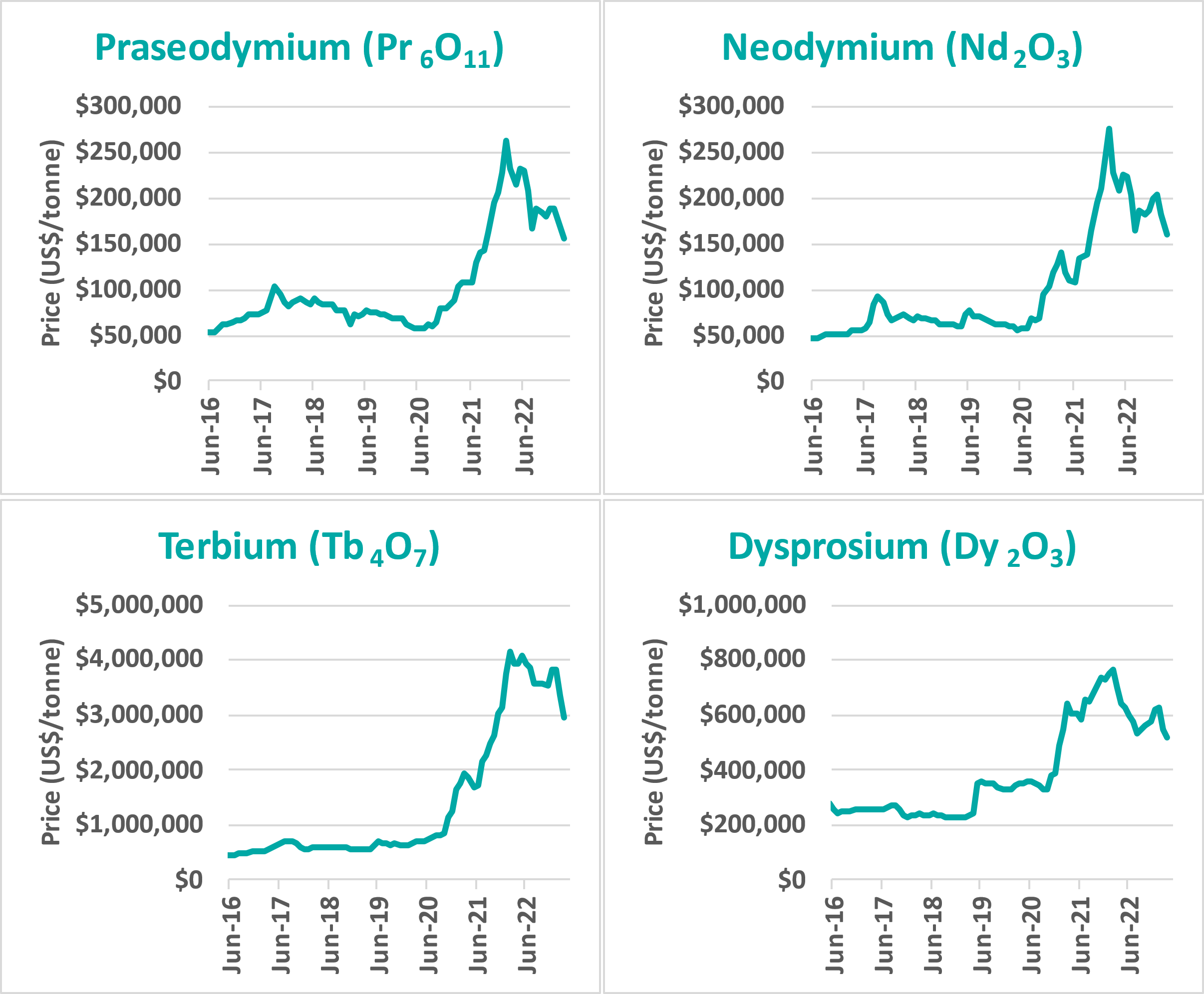
Figure 1: Prices for permanent magnet rare earths have increased significantly in the last two years (source: Kitco)
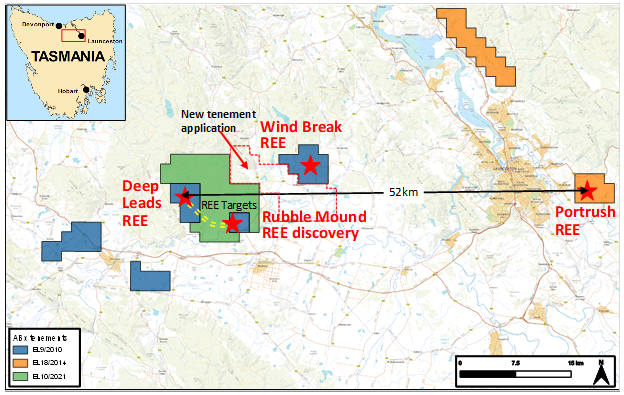
Figure 2: ABx leases in the 52 km wide REE province, including the new exploration licence application covering the area between Deep Leads and the Wind Break rare earths discovery located 16km ENE of Deep Leads.
Terminology
Rare earth elements (REE) consist of lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) and lutetium (Lu). Yttrium (Y) is also typically
grouped with the REE.
TREO
TREO are the total rare earth element oxides in the sample, with the REE metals expressed as rare earth element oxides, which is a common method for summarising the total grade.
TREO-CeO2
This is TREO minus the amount of cerium oxide in the sample. CeO2 is relatively low in value.
PPM
Ppm is parts per million by mass, which is the standard unit for reporting REE grades. 10,000ppm = 1.0%.
Permanent magnets
Permanent magnets are used in electronic and computing equipment, batteries, electric vehicles, wind
turbines, mobile phones and military systems. Nd & Pr are used in high-power permanent magnets. Dy, Sm and Tb are used in high-temperature permanent magnets. Some reporters called them “Super Magnet” REE.
Ionic adsorption clay (IAC) rare earth elements
In contrast with hard-rock REE ores, ionic adsorption clay REE mineralisation forms when REE attach loosely to clays and can be recovered by low-cost leaching methods.
IAC REE deposits have been mined in southern China and Myanmar. ABx is one of the very few listed
companies to discover true IAC REE mineralisation in Australia.
Extraction rates from desorption tests
To assess the potential of extracting REEs from prospects, tests have been carried out by ANSTO, who have extensive experience in metallurgical testing of clayhosted rare earth deposits worldwide. These were conducted at “standard” desorption conditions of 0.5 M ammonium sulfate at pH 4 which are low-acid, low-cost processing conditions for ionic adsorption clay REE.
The ”extraction rate” is the proportion of REE contained in the sample that is extracted and reports to the leach solution. Very few other REE occurrences in Australia have achieved extraction rates that have been achieved on ABx’s REE mineralisation in the channels at the Deep Leads project area in northern Tasmania.
Hydrogen Fluoride
Hydrogen fluoride is an essential chemical for the production of fluorocarbons and aluminium fluoride.
Hydrogen fluoride is mainly produced from fluorspar, which is obtained from the mineral fluorite. Fluorspar is relatively high cost and has been identified as a critical material by the USA, Europe, Japan and Canada.
Australia does not mine any fluorite, or produce any fluorspar or hydrogen fluoride and so must import all its requirements. The Australian demand for hydrogen fluoride is small, and it is imported at high cost.
Alcore has developed a world-first process to recover hydrogen fluoride from aluminium smelter bath. This can be combined with aluminium hydroxide to produce aluminium fluoride. Alcore is also investigating the use of dross (another aluminium smelter waste) and bauxite as alternatives to aluminium hydroxide as the source of aluminium. The use of dross or bauxite would further lower the production cost.
Alcore intends to construct commercial hydrogen fluoride plants in Bell Bay, Tasmania.
The initial plant is proposed to transform 1,600 tonnes per year of aluminium smelter bath into hydrogen fluoride and other industrial chemicals. A proportion of the hydrogen fluoride will be further processed to aluminium fluoride. The hydrogen fluoride produced can be optimised to suit market demand.
Aluminium Fluoride
Aluminium fluoride is an essential chemical for aluminium production.
Australia does not mine any aluminium fluoride, and so must import all its requirements. Australia is a significant producer of aluminium and so its demand for aluminium fluoride is high.
Australia is the largest producer of primary aluminium metal without its own domestic aluminium fluoride production, so Australian aluminium smelters rely entirely on imported aluminium fluoride. This is typically more than 80% from China, but this proportion was only 40% in 2021 when China production was lower, illustrating the supply risks (Figure 1). Aluminium fluoride prices have been above US$1,350/t for the last 16 months (Figure 2).
Most modern aluminium smelters produce excess bath, for which the only meaningful market is new smelters, which require bath to commence operations. Aluminium industry forecasts suggest that the global bath market will increasingly be in surplus, because far fewer new smelters are being constructed. All of the major global aluminium producers are eager for alternative applications for excess bath, to avoid the unpalatable options of on-site storage or landfill.
Alcore has developed a world-first process to recover hydrogen fluoride from aluminium smelter bath. This can be combined with aluminium hydroxide to produce aluminium fluoride. Alcore is also investigating the use of dross (another aluminium smelter waste) and bauxite as alternatives to aluminium hydroxide as the source of aluminium. The use of dross or bauxite would further lower the production cost.
Alcore intends to construct aluminium fluoride plants in Bell Bay, Tasmania. The aluminium source for the initial aluminium fluoride production is likely to be aluminium hydroxide, as this is less risk and allows a faster path to production. Subsequent production may use aluminium from dross or bauxite to further improve the financial and environmental outcomes.
The initial plant is proposed to transform 1,600 tonnes per year of aluminium smelter bath into hydrogen fluoride and other industrial chemicals. A proportion of the hydrogen fluoride will be further processed to aluminium fluoride. The aluminium fluoride produced can be optimised to suit market demand. Alcore’s longer term plan is to expand the plant by 15 times, which will process all of Australia’s aluminium smelter bath, and supply more than 80% of Australia’s aluminium fluoride requirements.
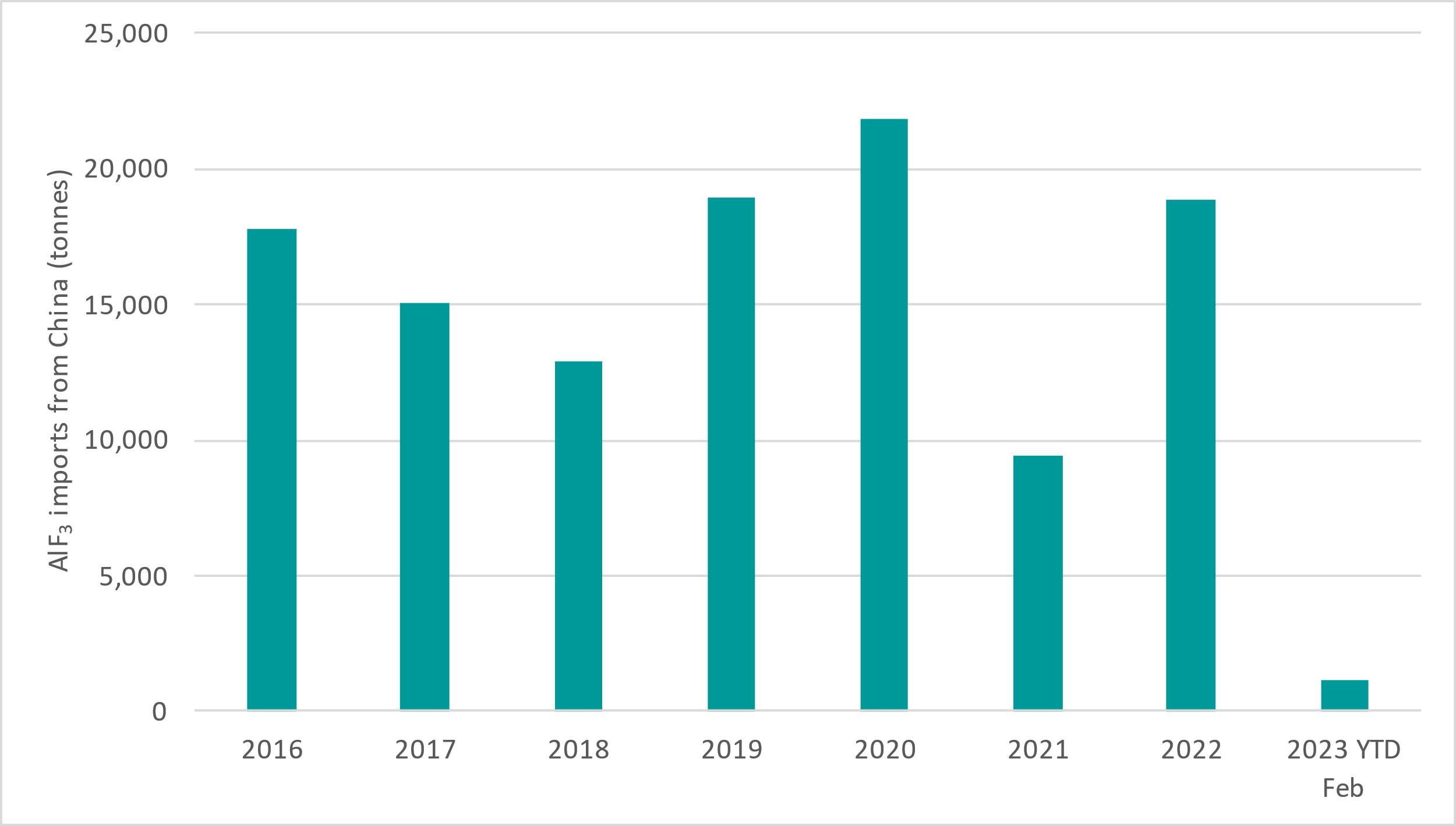
Figure 1: Imports of aluminium fluoride from China into Australia (source: China Customs Statistics)
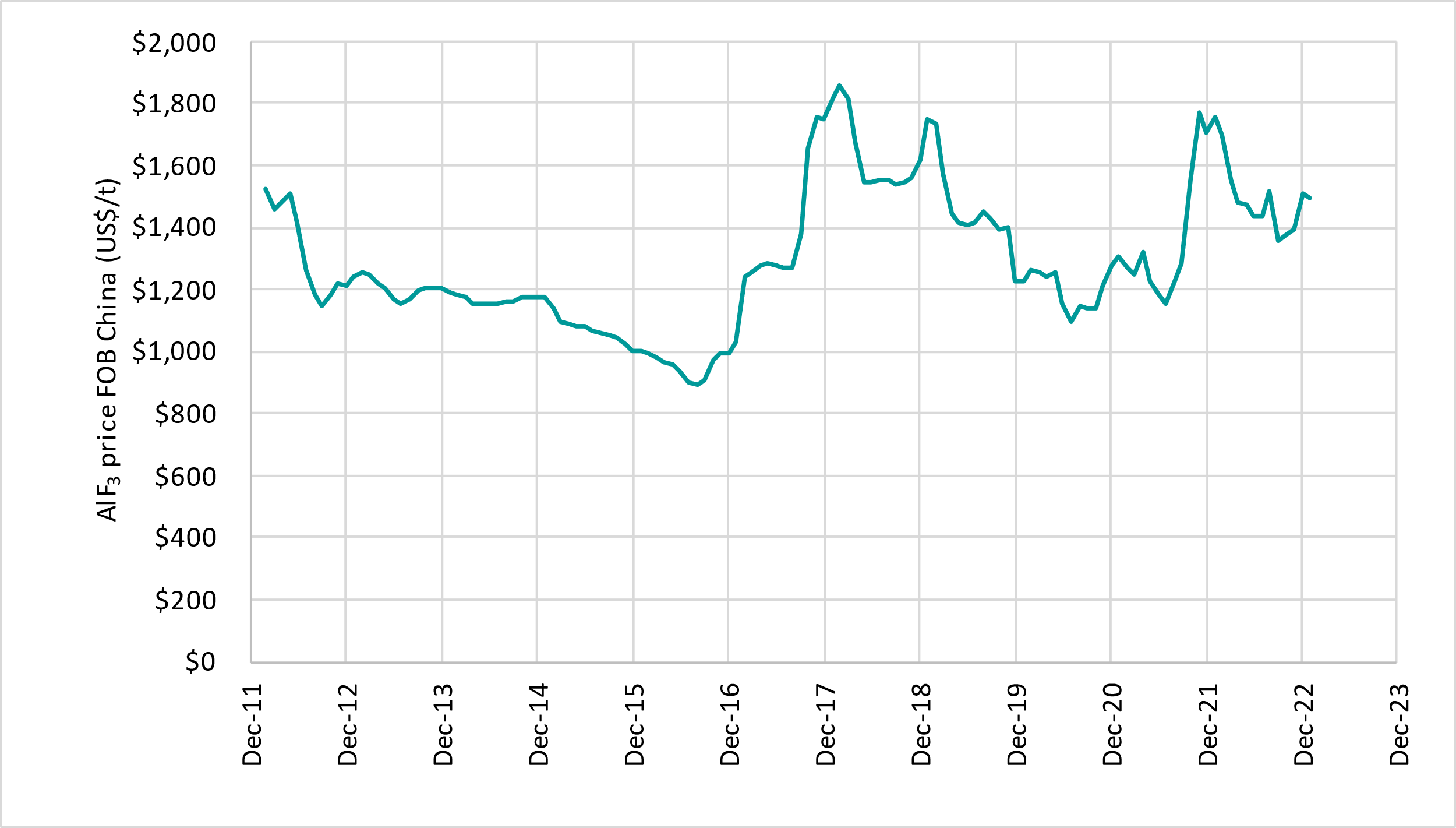
Figure 2: Aluminium fluoride monthly prices FOB China (source: China Customs Statistics)
Bauxite
Bauxite is a mainly a mixture of iron and aluminium hydroxides and oxides of Al, Fe, O and OH. Principal aluminium hydroxide minerals found in varying proportions are gibbsite and the polymorphs of boehmite and diaspore. Other components include SiO2, TiO2 and P2O5. The overall chemistry of bauxite determines its commercial usage.
Gibbsite has the chemical formula Al (OH)3, which is a trihydrate. Boehmite and diaspore have the formula AlO(OH), which is monohydrate. The main mineral of aluminium in our bauxite deposits is gibbsite. Large bauxite deposits in China and Vietnam are either low grade, high in monohydrate minerals, and/or high in reactive silica. Dissolution of monohydrate minerals requires higher temperatures and higher concentration of caustic soda; energy consumption is much higher than for dissolution of trihydrate gibbsite.
Dissolution of gibbsite requires lower temperatures and lower concentration of caustic soda, providing ABx with a significant competitive advantage.
Moreover, our bauxite deposits have low levels of reactive silica and high total alumina to silica ratios. Reactive silica is the most harmful contaminant because it forms insoluble double Al, Na silicate which reports to red mud during conversion to alumina.
High levels of reactive silica causes the loss of alumina and caustic soda, while TiO2 and P2O5 robs sodium. Our bauxite deposits are low in contaminants.
[1] ASX announcement 31 May 2022
[2] ASX announcement 2 February 2023